I recently enrolled in a clinical trial at Wake Forest Baptist Health. A phase I trial to test TeneoBio’s TNB-383B. Before this, I spent several months on carfilzomib, dex, and cyclophosphamide. Test results and bone marrow biopsy indicated I was relapsing.
TNB-383B is a BCMA x CD3 T-cell engaging bispecifc antibody being studied in relapsed or refractory multiple myeloma who have received at least 3 prior lines of therapy.
TNB-383B is being developed by TeneoOne through Phase 1. AbbVie holds the exclusive right to acquire TeneoOne and lead subsequent global development and commercialization of TNB-383B.
AbbVie, Inc. “TNB-383B.” AbbVie. Accessed November 24, 2020. https://www.abbvie.com/our-science/pipeline/tnb-383b.html.
I had one infusion of the drug almost two weeks ago. The first infusion required a hospital stay due to the potential for serious side effects, primarily cytokine release syndrome and tumor lysis syndrome. It sounds scarier than it was, in my case.
About two hours after the infusion of TNB-383B I began to experience an extreme skin sensitivity, aching joints — mostly knuckles and elbows, rigors, headache, and a fever of something over 103 degrees F. I’m not sure what the ultimate high temperature was. I had not known about rigors before this event. I don’t think I was shivering as much as what I’ve heard others talk about. I was extremely cold, and I think I was constantly begging for a blanket. I don’t really remember everything! I was aware at some point that they were talking about testing me for Covid-19, just to make sure that wasn’t the cause of the symptoms. They were also giving me fluids and Tylenol. I remember being wheeled to an isolation room, which was something they did as a precaution. In case I had Covid-19. My blood pressure also dropped about 30 points. I had a rapid heart rate, too. I heard a nurse talking about giving me morphine, which I declined. I’m not sure why I did that. Later I learned that morphine helps with rigors.
I could tell the efforts of the staff were beginning to be successful when I was no longer cold. Isn’t it weird that having a high fever would make me cold? Throughout the next few days, I was given fluids and Tylenol.
By the way, I was not positive for Covid-19. And, the swab test is not as bad as the crybabies on TV have reported. : ) I guess it’s all relative. If you’ve had bone marrow biopsies and bone fractures, no swab into the nasal cavity is going to bother you.
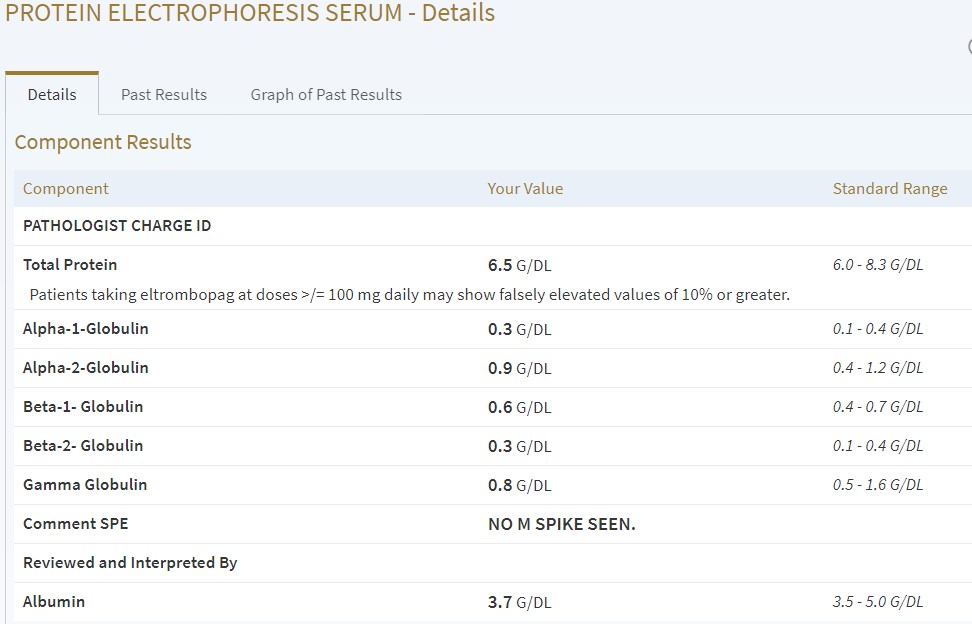
Next time, I’ll post some of the test results.
 It’s been a while since I took Pomalyst or Revlimid. I used to have to make a call to Celgene to take a survey. The purpose of the survey is just to make sure patients are aware of the safety concerns. I just got a call from the cancer center pharmacy that I can pick up my pom prescription when I go in on Thursday, as long as I’ve completed my survey. I searched for the online survey, and found out that they have an app now! It will remind me when my next survey is due.
It’s been a while since I took Pomalyst or Revlimid. I used to have to make a call to Celgene to take a survey. The purpose of the survey is just to make sure patients are aware of the safety concerns. I just got a call from the cancer center pharmacy that I can pick up my pom prescription when I go in on Thursday, as long as I’ve completed my survey. I searched for the online survey, and found out that they have an app now! It will remind me when my next survey is due.